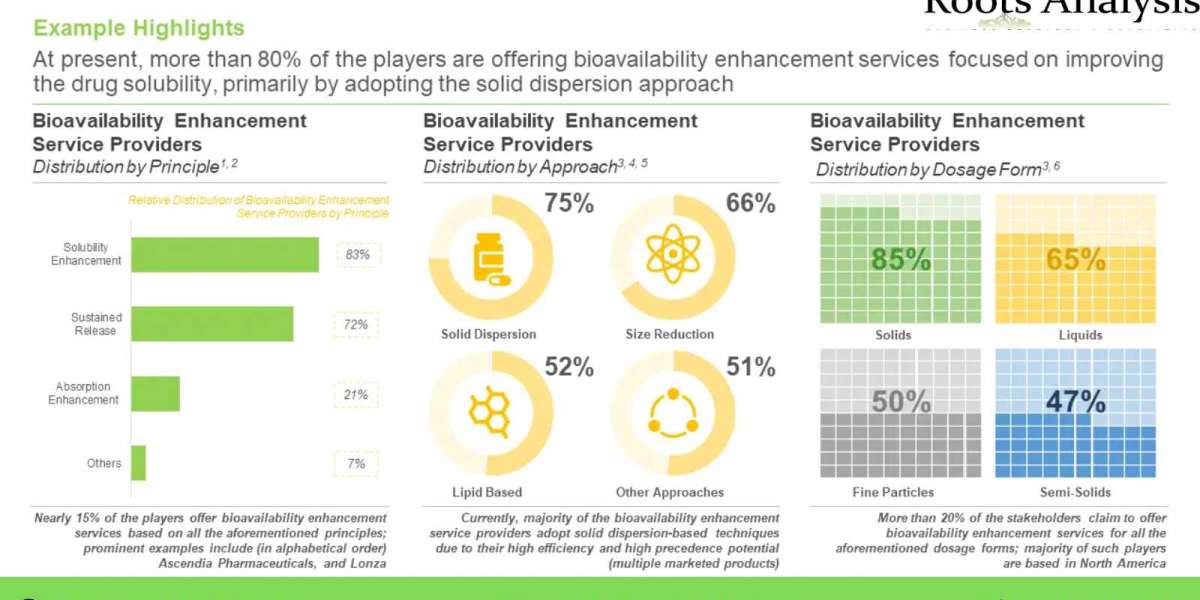
Moreover, it is a well-established fact that the systemic / local absorption and distribution of a therapeutic intervention is directly proportional to its bioavailability. In this context, it is also worth highlighting that more than 90% of new chemical entities (NCEs) developed by pharmaceutical companies and nearly 40% of the top-ranking oral drugs marketed in North America and Europe are insoluble in water and, hence, their low solubility can lead to deficient drug concentration, which further leads to in vivo failure. As a result, a large number of companies are now considering adopting various types of bioavailability enhancement technologies in order to ensure that their proprietary pharmacological product candidates are made bioavailable at optimal quantities at the desired site of action.
However, in addition to being a time and cost intensive process, bioavailability enhancement is often fraught with several challenges and considering the expertise available with specialty service providers to improve the pharmacokinetic properties of novel molecules, drug developers are actively outsourcing their requirements.
Diverse Landscape of Bioavailability Enhancement Technology and Service Providers
During our research, we came across nearly 115 players, which claim to offer technologies and services for bioavailability enhancement of drug products / candidates. As per our analysis, North America and Europe, with the highest number of technology as well as service providers, have emerged as key hubs. Further, across all regions, majority of the companies are well established.
Which Company has an Edge Over Other Companies?
Several bioavailability enhancement service providers are constantly making efforts to expand current capabilities to enhance their respective product portfolios and comply to evolving industry benchmarks.
Recent activity in Bioavailability Enhancement Market
At present, the bioavailability enhancement technology and service providers are actively trying to consolidate their presence in this field by entering into strategic alliances, to meet the indubitably rising demand for effective therapeutics. For this purpose, substantial mergers and acquisitions have been reported in this market, as service providers strive to become one-stop-shops, to cater to the diverse needs for their clientele.
Current Annual Demand for Bioavailability Technologies and Services
Considering the concerns related to low solubility / permeability of certain marketed drugs and a large number of NCEs, the demand for bioavailability enhancement technologies and services is anticipated to rise in the future, with a CAGR of ~10%.
Future Evolution of Bioavailability Enhancement Services Market
As mentioned earlier, owing to the high costs and technical expertise associated with formulation development process, stakeholders in the industry are likely to rely more on specialty service providers. Additionally, driven by the increase in number of BCS II and BCS IV molecules being evaluated in early phases of development, the bioavailability enhancement domain is likely to grow at CAGR of ~11%, till 2035
Further, the estimated market opportunity is expected to be well distributed across different types of drug classes, dosage forms and key geographical regions
Technology Evaluation Framework
With the market evolving at a steady pace, emerging players need to incorporate innovative bioavailability enhancement technologies to augment their service portfolio and surpass the competition. To address these concerns, we have proposed a proprietary framework.
Technology evaluation framework provides a value addition matrix for bioavailability enhancement approaches currently employed by industry stakeholders. The framework highlights the implementation of several advanced as well as traditional bioavailability enhancement approaches and technologies at different stages of the drug development pathway. Further, it provides a detailed analysis on ease of implementation and associated risk in integrating such technologies, based on technology efficacy / success rate, approved drugs, extent of innovation, global competition, and trends in research activity.
For additional details, please visit
https://www.rootsanalysis.com/blog/bioavailability-enhancement-technologies/ or email sales@rootsanalysis.com
You may also be interested in the following titles:
- Smart Labels Market: Industry Trends and Global Forecasts, 2022-2035
- 4D Bioprinting Market : Industry Trends and Global Forecasts, 2022-2035
About Roots Analysis
Roots Analysis is a global leader in the pharma / biotech market research. Having worked with over 750 clients worldwide, including Fortune 500 companies, start-ups, academia, venture capitalists and strategic investors for more than a decade, we offer a highly analytical / data-driven perspective to a network of over 450,000 senior industry stakeholders looking for credible market insights.
Contact:
Ben Johnson
+1 (415) 800 3415
Ben.johnson@rootsanalysis.com