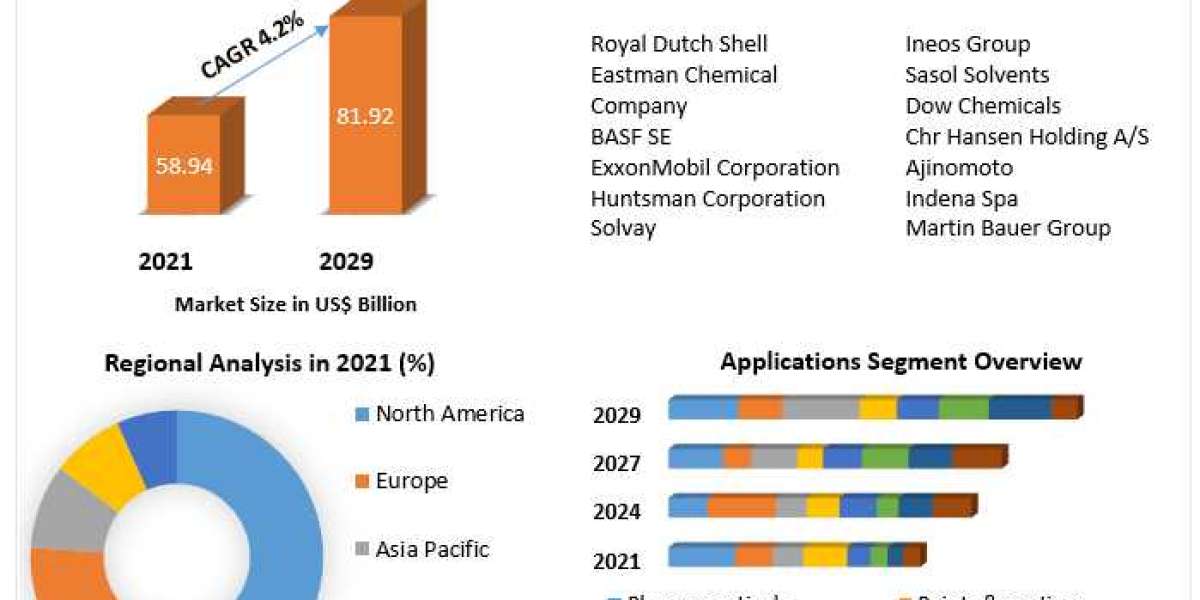
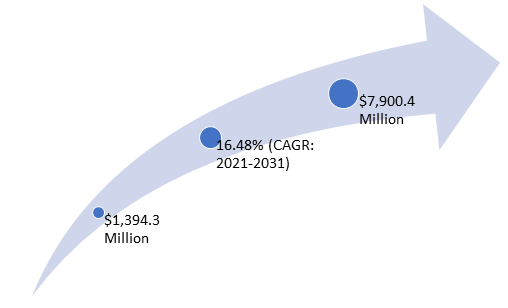
The use of infectious disease diagnostics kits, assays, and other consumables has increased due to the rising prevalence of cancer, the increasing key player initiatives, and the rising government initiatives. The increasing investments by healthcare companies to meet industry demand and the growing adoption of infectious disease diagnostics kits, tubes, and reagents among major end users are major factors propelling the growth of the Europe infectious disease diagnostics market. The Europe infectious disease diagnostics market was valued at $1,394.3 million in 2020 and is expected to reach $7,900.4 million by 2031, witnessing a CAGR of 16.48% during the forecast period 2021-2031.
Competitive Landscape
The Europe infectious disease diagnostics market comprises well-established and newly emerging companies. Several companies are attempting to sustain their position in the market by launching new products and raising funds to develop new products for infectious disease diagnostics from different product types. Key market players of the Europe infectious disease diagnostics market witnessed product launch and approval, business funding, and synergistic activities from January 2018 to March 2022. The inclination of companies toward product launches suggests that companies are constantly bringing new products to the market to test viral and bacterial infections, which is primarily attributed to an increase in demand for kits and assays by end users.
Market Segmentation
• Product (system, kits and reagents, and software)
• Testing location (laboratory testing and point-of-care testing)
• Technology (next-generation sequencing (NGS), polymerase chain reaction (PCR), immunodiagnostics, isothermal nucleic acid amplification technology (INAAT), microarray, in-situ hybridization (ISH), and other technologies)
• Infectious Disease Type (bacterial infectious diseases, viral infectious diseases, fungal infectious diseases, and other infectious diseases)
• Infection Type (respiratory infections, hospital-acquired infections (HAI), sexually transmitted infections (STIs), and other infections)
• End User (hospitals, diagnostic centers, out-patient clinics/general practitioners, research laboratories, and other end users)
Regional Segmentation
• Europe-5 - Germany, U.K., France, Italy and Spain
• Nordic- Denmark, Sweden, Norway and Finland
• Baltic- Estonia, Lithuania and Latvia
• Rest-of-Europe
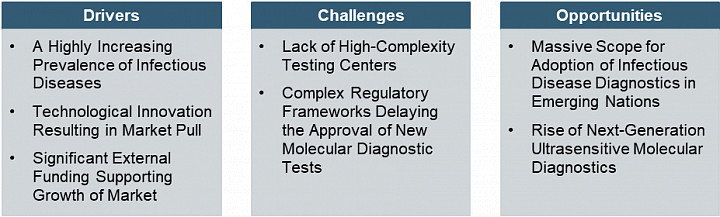
Europe Infectious Disease Diagnostics Market Dynamics
Key Questions Answered in this Report:
• How is each segment of the market expected to grow during the forecast period 2021-2031, and what is the anticipated revenue to be generated by each segment? Following are the segments:
o Product (system, kits and reagents, and software)
o Testing location (laboratory testing and point-of-care testing)
o Technology (next-generation sequencing (NGS), polymerase chain reaction (PCR), immunodiagnostics, isothermal nucleic acid amplification technology (INAAT), microarray, in-situ hybridization (ISH), and other technologies)
o Infectious Disease Type (bacterial infectious diseases, viral infectious diseases, fungal infectious diseases, and other infectious diseases)
o Infection Type (respiratory infections, hospital-acquired infections (HAI), sexually transmitted infections (STIs), and other infections)
o End User (hospitals, diagnostic centers, out-patient clinics/general practitioners, research laboratories, and other end users)
o Region (EU-5, Baltic, Nordic, and Rest-of-Europe)
• What are the major market drivers, challenges, and opportunities in the Europe infectious disease diagnostics market?
• What are the underlying structures resulting in the emerging trends within the Europe infectious disease diagnostics market?
• How is each segment of the Europe infectious disease diagnostics market expected to grow during the forecast period, and what will be the expected revenue generated by each of the segments by the end of 2031?
• What are the key developmental strategies implemented by the major players to sustain in the competitive market?
• What are the key regulatory implications in developed and developing regions for molecular diagnostics?
• Who are the leading players with significant offerings to the Europe infectious disease diagnostics market? What is the current market dominance for each of these leading players?
• What would be the compound growth rate witnessed by the leading players in the market during the forecast period 2021-2031? Which infectious disease product type is anticipated to have the most promising growth?
• What are the key applications in the Europe infectious disease diagnostics market? What are the major segments of these applications? What technologies are dominating these application segments?
• What are the major technologies that are employed in the Europe infectious disease diagnostics market? Which is the dominating technology?
• Who are the primary end users of the Europe infectious disease diagnostics market? Which is the fastest-growing end-user segment in the Europe infectious disease diagnostics market?
• Who are the key manufacturers in the Europe infectious disease diagnostics market, and what are their contributions? Also, what is the growth potential of each major infectious disease diagnostics manufacturer?
• What is the scope of the Europe infectious disease diagnostics market in the EU’5, Baltic, and Nordic regions? Which infectious disease diagnostics product and end user dominate these regions?
• What are the emerging trends in the Europe infectious disease diagnostics market? How are these trends revolutionizing the diagnostic procedure?
• Which technologies are anticipated to break through the current infectious diseases diagnostic regime?
• Which companies are anticipated to be highly disruptive in the future and why?
• What are the regulatory procedures required to unify the approval process for emerging infectious disease diagnostics? How will these enhance the reimbursement scenario?
• What are the gaps in regularizing optimum infectious disease diagnostics adoption in regular healthcare routines? How are these gaps being tackled?
Request Sample - https://bisresearch.com/requestsample?id=1294type=download
BIS Related Studies