However, the surge in the demand for drugs due to COVID-19 has led the pharmaceutical industry to shift on to a more agile and flexible technique- continuous, which is a single flow process allowing the industry to scale up quick, manufacture easily and shortens the drug supply chain. In fact, the USFDA has categorized continuous as one of today’s most important tools for modernizing the pharmaceutical industry.
Advantages of Continuous Manufacturing
Some of the key advantages are highlighted below:
- Reduction in manufacturing cost (by 15-30%)
- Reduction in manpower (by 50-70%)
- Lower product deviation (by 50%)
- Smaller footprint requirement (by 50-70%)
- Reduction in power consumption (by 40%)
- Faster scale up.
Moreover, the continuous processes use advanced sensors, and in-process analytics that enable the measurement of critical parameters and processing conditions in real-time.
List of Companies Offering Continuous Manufacturing
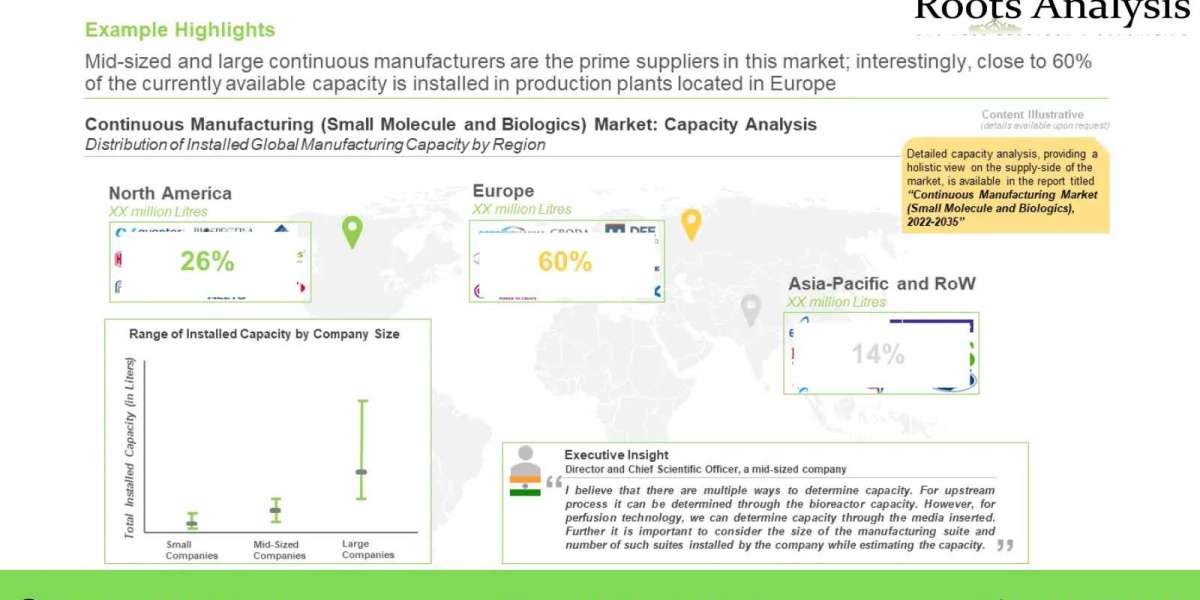
Currently, over 70 companies across the globe claim to offer continuous manufacturing services. Over 90% of the companies are engaged in offering services for API manufacturing, while only 53% of the companies offer the services related to final dosage form. Furthermore, it is interesting to note that 49% of the continuous manufacturing players are predominantly present in Europe. There has been a significant increase in the number of expansions in order to adopt continuous manufacturing since 2016. Majority of expansions were signed in the Europe (primarily in Germany and United Kingdom). Moreover, close to 50% instances were related to plant establishment followed by continuous manufacturing technology enhancement (30%).
Adoption of Continuous Manufacturing
As per the interviews conducted with industry stakeholders, the industry is currently in the initial stages of adopting continuous manufacturing. However, the ongoing COVID-19 crisis is likely to accelerate the industry’s shift to these systems. In fact, a number of established big pharma players have already adopted continuous manufacturing system.
For additional details, please visit https://www.rootsanalysis.com/blog/continuous-manufacturing-a-magic-bullet-to-meet-the-demand-for-pharmaceutical-products/ or email sales@rootsanalysis.com
You may also be interested in the following titles:
- Flow Cytometry Service Market, 2022-2035
- Gene Editing beyond CRISPR Market, 2022-2035
About Roots Analysis
Roots Analysis is a global leader in the pharma / biotech market research. Having worked with over 750 clients worldwide, including Fortune 500 companies, start-ups, academia, venture capitalists and strategic investors for more than a decade, we offer a highly analytical / data-driven perspective to a network of over 450,000 senior industry stakeholders looking for credible market insights.
Contact Information
Ben Johnson
+1 (415) 800 3415
+44 (122) 391 1091