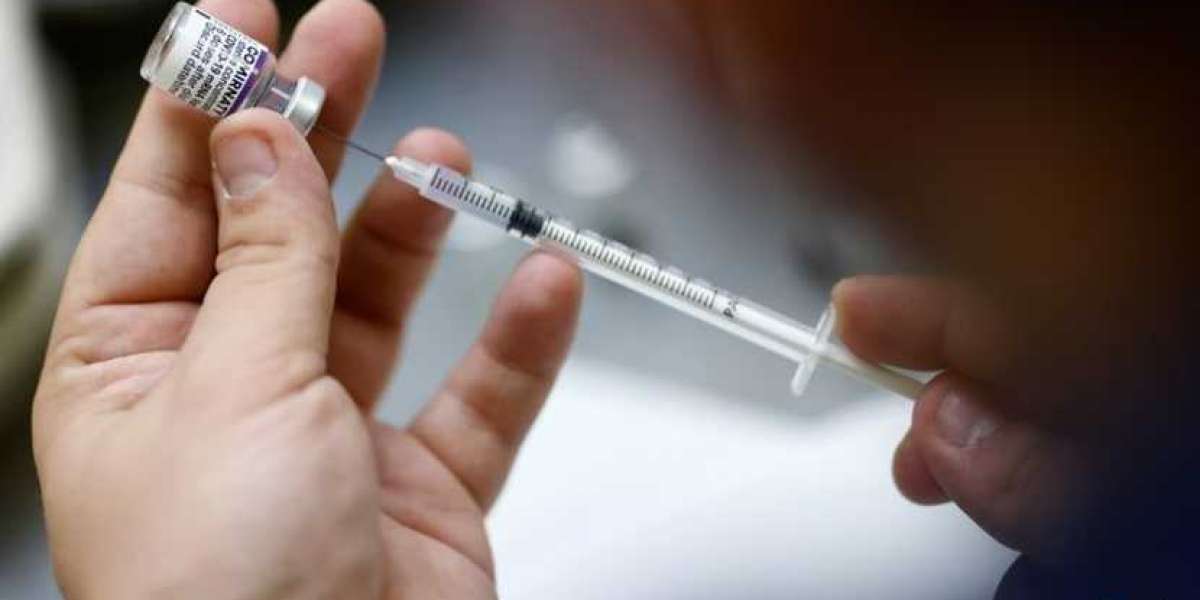
The director of the US Centers for Disease Control and Prevention (CDC) on Tuesday (Nov 2) backed broad use of Pfizer-BioNTech's COVID-19 vaccine in children aged five to 11, clearing the way for shots to go into young arms as soon as Wednesday.
What friends get for a pre-trial trial is that they'll get used to XO and know what they know about. Of course, when friends know this,
The announcement comes hours after the advisers to the US CDC unanimously supported the move, saying the benefits of the vaccine outweigh the risks. Much of their discussion stemmed from rare cases of heart inflammation that have been linked to the vaccine, particularly in young men.
The US Food and Drug Administration (FDA) granted emergency use authorisation of the vaccine in five- to 11-year-olds on Friday.
The FDA authorised a 10-microgram dose of Pfizer's vaccine in young children. The original shot given to those aged 12 and older is 30 micrograms.
"We know millions of parents are eager to get their children vaccinated and with this decision, we now have recommended that about 28 million children receive a COVID-19 vaccine," CDC director Rochelle Walensky said in the statement.
At the outset of the meeting, Walensky said that paediatric hospitalisations had surged during the recent wave driven by the Delta variant of the coronavirus.
The risk from COVID-19 "is too high and too devastating to our children and far higher than for many other diseases for which we vaccinate children", she said.