The process by which one atom consumes another atom is referred to as absorption. Spectroscopy is a type of instrumental analysis that can relatively quickly determine whether or not a sample contains trace metals. This can be done to determine whether or not a sample contains trace metals. It is also utilized in many other fields, such as forensic science, which is just one of those fields. This technology also has a number of other applications, including the analysis of trace metals in pharmaceuticals, foods, and beverages; geological and mineralogical samples; biological fluids and specimens; and other types of samples. Recent advancements have helped contribute even further to the overall improvement, which is largely due to the contribution that these advancements have made. It is possible that trace metals are present, but it is also possible that they are not present at all. Both possibilities are open to consideration.
The chemical techniques that are used for the analysis of trace metals have evolved from simple gravimetric methods to highly sophisticated and effective instrumental techniques. Previously, these techniques were only used. These techniques were utilized at one point in time in the past. In the past, in order to finish all of these steps, a significant amount of additional time had to be invested in them.
The Primary Ideas That Are Responsible for Laying the Groundwork for Future Developments in Atomic Absorption Spectroscopy
1. Atomic absorption spectroscopy, also known as AAS for short, is a method of spectroscopy that is based on the concept that free atoms that have transitioned back to their ground states are able to absorb light of a specific wavelength
2. This concept is the foundation for the AAS method
3. This guiding principle forms the foundation of the method and serves as its primary constituent
An investigation into extremely minute quantities of environmental pollutants
Researchers have been successful in identifying trace metals present in a wide variety of matrices and making accurate quantitative assessments of the concentrations of these metals by employing a wide variety of distinct analytical techniques. This has allowed the researchers to make accurate measurements of the concentrations of the trace metals.
The method of instrumental analysis known as atomic absorption spectroscopy produces results with a high degree of accuracy and precision at a cost that is comparable to that of other methods. Atomic absorption spectroscopy is a technique that can be utilized in the analysis of a wide variety of different substances. It is a method that was developed in the 1950s. Each and every one of these classifications of research facilities makes use of it.
Atomic absorption spectroscopy, also referred to as AAS, is a technique that has a wide range of potential applications. Examining the make-up of various rocks and minerals is the best way to obtain this kind of knowledge. Discovering this information may be possible through the exploration of regions that have already been investigated. When searching for oil and water deposits, it is very helpful to conduct an analysis of trace metals because atomic absorption spectrophotometer can help identify potential locations. This can help narrow down the search.
Maintaining a State of Alertness Regarding the Natural WorldMonitoring the environment for the presence of trace metal contamination in industrial effluents, oceans, rivers, and lakes is essential in order to determine whether or not the water is safe for drinking and other commercial applications. Trace metal contamination can be found in all of these bodies of water. It has been discovered that even minute amounts of metal contamination can be found in each of these bodies of water.
Materials DevelopmentThe composition of the material as well as the trace metals that are present in the material can have a significant impact on the properties of the material, such as the material's hardness, brittleness, grain size, crystallinity, and amorphous nature. These properties include: hardness, brittleness, grain size, crystallinity, and amorphous nature. Because consumers are becoming more aware of food safety issues on a daily basis, manufacturers are under increasing pressure to ensure that the levels of trace metals in their products do not exceed the permissible limits. This is because consumers are becoming more aware of food safety issues on a daily basis. This is because consumers are increasing their awareness of concerns regarding the safety of food. This is as a result of the fact that consumers are continually becoming more aware of concerns regarding the quality and safety of the food they consume. On the other hand, the completion of this step is not required in any of the processes involved in the production of petroleum products.
The study of atomic absorption spectroscopy encompasses a wide variety of subfields and applications, each of which is extremely unique.
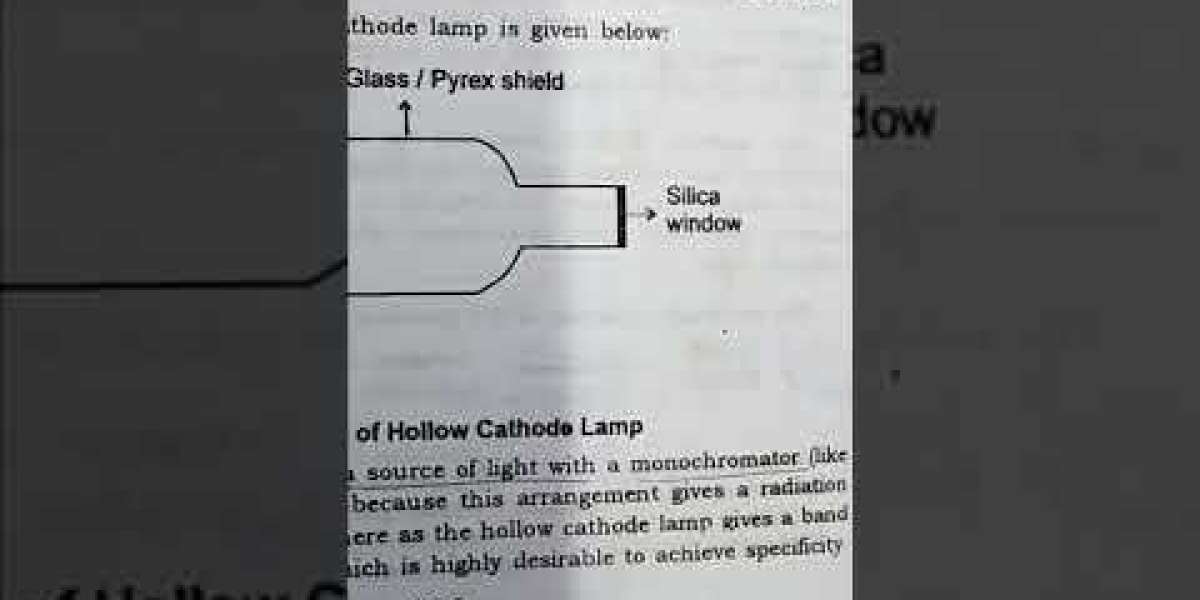
Atomic absorption spectrometry (AAS) systems are one example of laboratory equipment that can be purchased at prices that are more reasonable than they were in the past. These days, these systems can be found all over the world. This marks the beginning of the process in its entirety. Atomization is a necessary step in the AAS process because it contributes to the determination of the level of sensitivity that can be attained during the reading. This is one of the reasons why atomization is a necessary step in the AAS process. When an analyte is excited by line sources, the analyte will emit a line spectrum that is unique to the analyte as a result of this process.